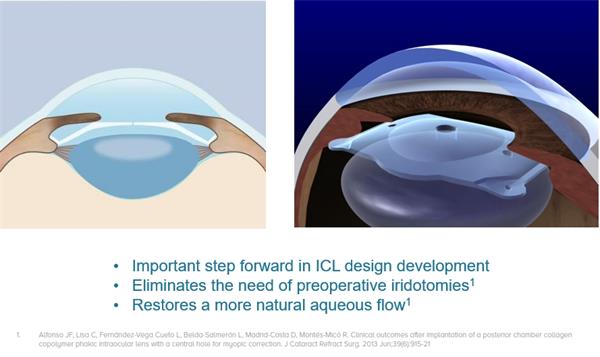
2018年,国内分别上市了VICMO和VTICMO两款产品(具体如下表所示),其光学直径为4.9~5.8mm,相较于VICMO和VTICMO,VICM5和VTICM5有更大的光学区,其光学直径为5.0~6.1mm,晶体屈光度-0.5D到-14.0D。
近年来临床应用反馈,VICM5对于大瞳孔患者的临床效果及在改善术后夜视力,潜在减少术后眩光及光晕等方面优于VICMO。
自2015年12月16日VICM5和VTICM5获得CE证书后在欧洲各国、日本、韩国及香港地区陆续上市并广泛应用于临床,***近几年(2015.12-2021.7)全球销量VICM5为178,629枚;VTICM5为136,141枚。其******性安全性得到充分验证,在不同人种的临床效果未见显著性差异。
EVO+ ICL产品介绍
EVO+ ICL产品型号

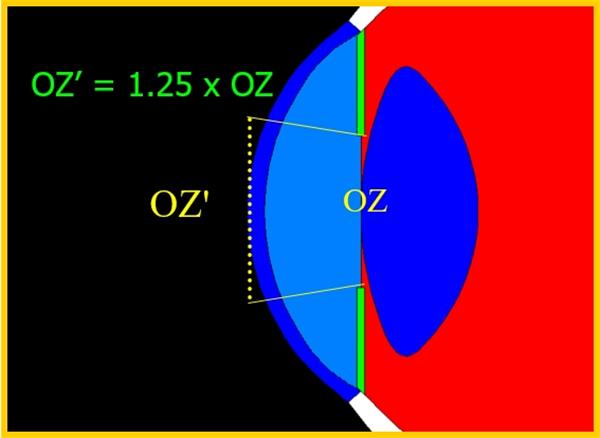
ICL光学区
EVO+产品革新

相比较于现有产品,V5有助于减少患者在术后可能发生的眩光光晕等主观视觉异常,可进一步提高患者的夜视力体验,能满足大瞳孔的屈光不正的患者及对视觉质量有极高要求的屈光不正患者,提高患者的满意度。
EVO+(VICM5 & VTICM5) 全球应用概况
EVO+(VICM5 & VTICM5) 2011年3月25日获得CE证书后在欧洲各国、日本、韩国及香港地区陆续上市并广泛应用于临床
EVO+产品特点
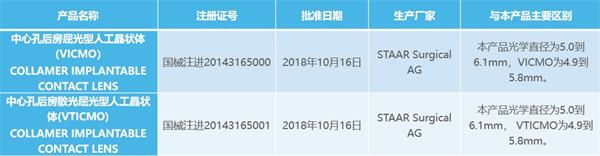
1、不切除任何角膜组织(1,2)
No corneal tissue removal – Great for thin corneas(1,2)
2、视觉质量高1,出色的夜间视力(3),不会引起干眼(4)
Vision Quality – Improved Visual Acuity1, Excellent Night Vision3, No induction of dry eye syndrome4
3、Collamer材料制成,超高生物相容性(5,6)
Made of Collamer - Highly biocompatible, inhibiting inflammatory responses to achieve a postoperative quiet (5,6)
4、超过1,000,000例的全球植入量
Proven track record – over 1,000,000 lenses implanted globally
5、提供紫外线保护
UV Protection
6、临床报道中患者满意度高(7)
Reported High Patient Satisfaction in a clinical study-(7)
可潜更大的光学区
在减少患者在术后可能发生的眩光光晕等主观视觉异常
更佳的夜视力体验
可满足大瞳孔的屈光不正的患者及对视觉质量有极高要求的屈光不正患者
1.Parkhurst G, Psolka M, Kezirian G. Phakic intraocular lens implantation in United States military warfighters: A retrospective analysis of early clinical outcomes of the Visian ICL. J Refract Surg. 2011;27(7):473-481.
2.Gimbel H, et al. Management of myopic astigmatism with phakic intraocular lens implantation. Journal of Cataract & Refractive Surgery, Volume 28, Issue 5 , 883 – 886.
3.Parkhurst G. A prospective comparison of phakic Collamer lenses and wavefront-optimized laser-assisted in situ keratomileusis for correction of myopia. Clinical Ophthalmology. 2016.
4.Naves J, Carracedo G, Cacho-Babillo I. Diadenosine nucleotide measurements as dry-eye score in patients after LASIK and ICL surgery. Presented at American Society of Cataract and Refractive Surgery (ASCRS) 2012.
5.Schild G, Amon M, Abela-Formanek C, et al. Uveal and capsular biocompatibility of a single-piece, sharp-edged hydrophilic acrylic intraocular lens with collagen (Collamer): 1-year results. J Cataract Refract Surg. 2004 Jun;30(6):1254-8.
6.Brown D, Ziémba S. Collamer IOL FDA Study Group. Collamer intraocular lens: clinical results from the US FDA core study. J Cataract Refract Surg. 2001 Jun;27(6):833-40.
7.MICL PMA data: P030016.

Copyright © 2023 shiyanaier.com
鄂ICP备18010121号 鄂公网安备42030202000612号
(十)医广[2024]第05-10-038号